Sodium Cyanide is a highly toxic inorganic chemical compound. It is a white colour solid crystalline chemical which has a faint almond-like odour. It is a hygroscopic compound that emits hydrogen cyanide in damped condition. Sodium cyanide is formed when hydrogen cyanide reacts with sodium hydroxide.
HCN + NaOH → NaCN + H2O
Let’s know more chemical details of Sodium Cyanide.
Properties Of Sodium Cyanide
| Chemical formula | NaCN or CNNa |
| Molecular weight | 49.008 g/mol |
| Density | 1.5955 g/cm3 |
| Chemical names | Cyanogran
Cymag Cyanide of sodium |
| Boiling point | 1,496 °C |
| Melting point | 563.7 °C |
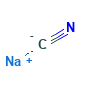
Sodium Cyanide Structural Formula
The structural formula for Sodium Cyanide is as shown in the figure below. It contains Sodium Cyanide contains equal numbers of sodium cations and cyanide anions.
Stay tuned with BYJU’S for more scientific information about chemical components!!
Comments